

#PERIODIC TABLE OF ELEMENTS WITH BLOCKS SERIES#
Let us look at the electronic configuration of transition series according to the Aufbau principle and Hund’s rule of multiplicity.Īnomalies in all the series can be attributed to the following reason: Half-filled orbitals and filled d orbitals can provide stability to these elements. The general configuration of D block elements is (n - 1)d 1-10ns 1-2. Elements like zinc and mercury have filled d subshells and are not considered transition metals.Īll transition metals are d block elements but not all d block elements are transition metals.Įlectronic Configuration of D Block Elements However, scandium and yttrium of group three are also considered transition metals due to their partially filled d subshell in the metallic state. The main groups of transition elements are four to eleven. Why Are D Block Elements Called Transition Metals?ĭ block elements have properties and position that transition between s and p block elements. Given below is a list of d block elements: Group 12 does not have elements in which electrons fill the d orbital, but they are still grouped in the d block because of similar chemical properties. The d block elements are those elements that have electrons (1-10) filled in the d orbital of the penultimate energy level and also in the s orbital (1-2) are classified as D block elements. D block elements are also known as transition elements or transition metals.
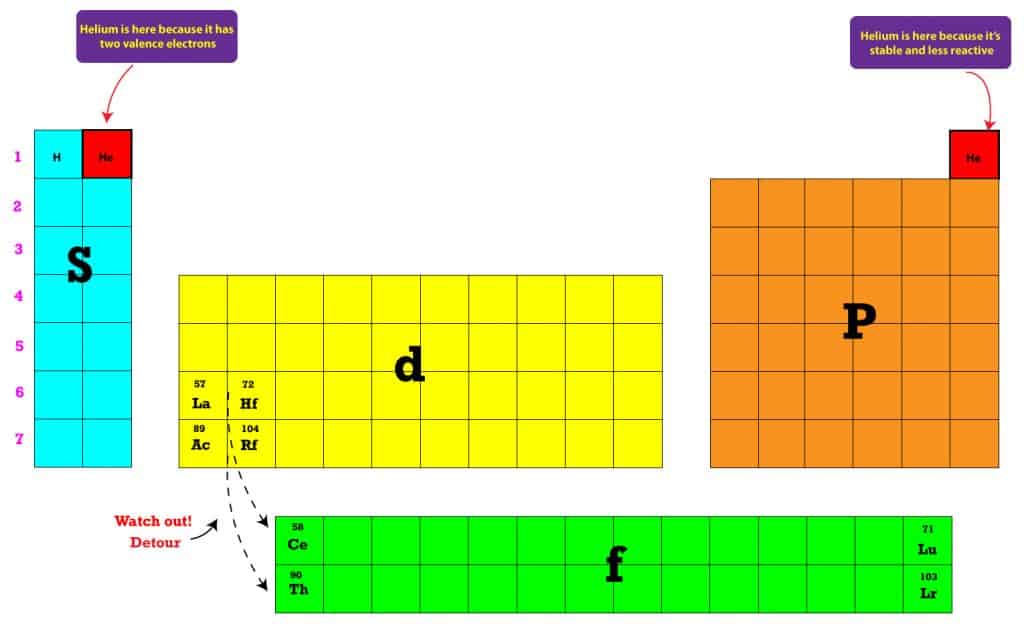
Their valence electrons are placed in the d orbital. D block elements are found on the modern periodic table from the third to the twelfth group.


 0 kommentar(er)
0 kommentar(er)
